Shared research databases help match more patients to cancer clinical trials
October 8, 2019
Shared research databases help match more patients to cancer clinical trials
Summary
This article describes a new approach to quickly match the right patient to a cancer clinical trial looking for patients with specific traits. The authors discuss shared research databases and how this new approach helps overcome challenges of precision medicine
in cancer clinical trials.
What is the new approach?
A network of researchers, healthcare providers, drug companies, and patient advocacy groups work together to create large, organized research databases of patient information and cancer samples.
When researchers must find patients with specific cancer traits, they can quickly and easily search the database for patients who match.
What is precision medicine and how has it changed clinical trials?
Precision medicine uses targeted therapies, which work by targeting a cancer’s specific genes, proteins, or traits related to cancer growth (also called biomarkers). Targeted therapies work best to treat patients known to have specific biomarkers.
Researchers carry out clinical trials to learn if new treatments are safe and work better than current treatments. Clinical trials of targeted therapies must find patients whose cancers have the specific biomarker that the therapy targets. But, researchers have trouble quickly finding the right patient with a specific biomarker to join their clinical trial.How do shared research databases work?
The network that runs each shared research database has a process to collect data and give researchers access to the data, which usually involves:
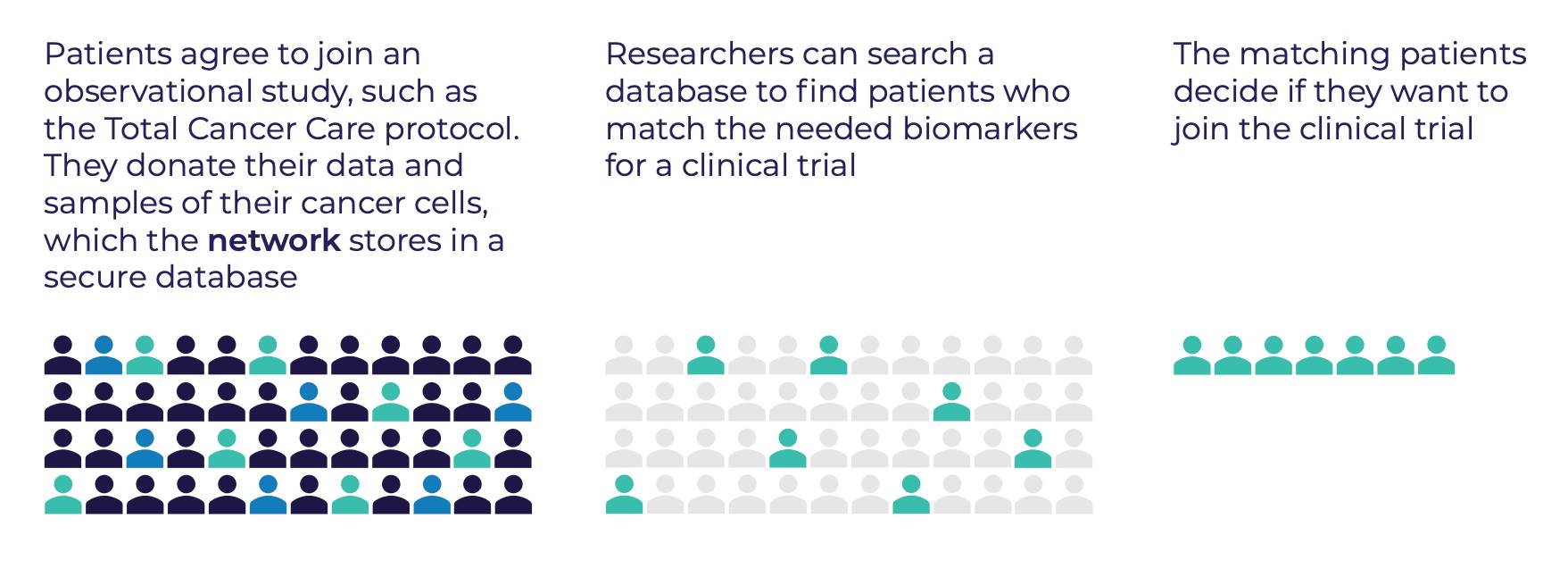
- Patients agree to join (consent to) an observational study, which is a type of study where researchers observe (watch and look at) outcomes but don’t assign any treatment
- As part of the observational study, patients donate their cancer data
- The network securely stores the data in the shared research database
- Researchers search this database to find patients with specific biomarkers and match them to clinical trials that use targeted therapies
For example, one network with a shared research database is Oncology Research Information Exchange Network (ORIEN). This is a unique research partnership among North America’s top cancer centers that uses an approved protocol with patient consent called Total Cancer Care® (TCC) to organize patients’ data.
-
What is precision medicine and how has it changed clinical trials?
Research has helped scientists better understand how the body works and discover new treatments. Precision medicine uses targeted therapies, which work by targeting a cancer’s specific genes, proteins, or traits related to cancer growth (also called biomarkers). Targeted therapies work best to treat patients known to have specific biomarkers. Since every cancer is unique, some cancers have a specific biomarker while many don’t.
Clinical trials help researchers improve cancer care. Through clinical trials, researchers learn if new treatments are safe and work better than current treatments. To carry out clinical trials of targeted therapies, researchers must find patients whose cancers have the specific biomarker that the therapy targets.
However, researchers have trouble quickly finding the right patients with a specific biomarker to join their clinical trial. It is often unknown if a patient has a specific biomarker at the point when a patient is being considered to join a clinical trial. While researchers can test for biomarkers in blood tissues or other body fluids at that point, this can delay a patient getting into the clinical trial and slow down the progress of the trial. That can mean delays in treatment for patients who urgently need it and higher costs for researchers. The impact could be even more significant if a certain biomarker is rare because in those cases researchers need to look at a large number of patients to complete a clinical trial.
What kinds of data do patients donate to a shared research database?
After a patient consents, the database stores a patient's clinical information for future research, such as:
- Samples of their cancer cells or tissues (which can be tested for biomarkers and DNA)
- De-identified genetic information
- Type of cancer
- Treatments they've had
- Outcomes, such as survival
-
What is the new approach to matching patients to cancer clinical trials?
A network of researchers, healthcare providers, drug companies, and patient advocacy groups work together to create large, organized research databases of patient information and cancer samples.
When researchers must find patients with specific cancer traits, they can quickly and easily search the database for patients who match. For example, they can search for patients whose cancers have a specific biomarker that a new treatment targets.
-
What kind of research is the new approach?
The new approach is a type of observational study, which means that researchers observe (watch and look at) outcomes but don’t assign any treatment. The big idea is that patients agree to join and donate their information to the database. The researchers can access the data over time for their clinical trials.
-
How does the observational study work?
First, patients enter an observational study and agree to donate information about their cancer, treatments, and cancer cells. This provides researchers with the genetic information needed to identify which patients are the best fit for clinical trials.
Then, healthcare providers and researchers group the patients into groups based on the information they provide. These groups are called cohorts or communities.
Finally, with a database of communities, healthcare providers can anticipate patients’ treatment needs and learn from their experiences.
-
How do shared research databases work?
The network that runs each shared research database has a process to collect data and give researchers access to the data, which usually involves:
- Patients agree to join (consent to) an observational study
- As part of the observational study, patients donate their cancer data and samples of their cancer cells
- The network securely stores the data in the shared research database
- Researchers search this database to find patients with specific biomarkers and match them to clinical trials that use targeted therapies
- Once matched, a patient decides if they want to join the clinical trial for a possible new treatment option
- If many clinical trials show that the treatment works and is safe to use, the treatment can be approved for use to treat cancer in people with the specific biomarker
-
What are some of the existing research databases?
Several networks have already formed to create shared research databases. While they may have different names for their databases, such as a registry or exchange, they all have the same big idea: a large, shared database with information and samples from thousands of cancer patients.
Oncology Research Information Exchange Network (ORIEN)
- A unique research partnership among North America’s top cancer centers that uses Total Cancer Care® to organize patients’ data, first established by the Moffitt Cancer Center in 2006A unique feature of the ORIEN Total Cancer Care® Protocol is that the patients consent to being followed throughout their lifetime for information about their cancer care. Researchers are able to access a dynamic database as patients’ genetic and clinical data are being updated on an ongoing basis.
- Learn more about ORIEN at http://www.oriencancer.org/
Targeted Agent and Profiling Utilization Registry (TAPUR)
- A clinical trial for people whose cancer no longer responds to standard (usual) treatment or has no standard treatment available, sponsored by the American Society of Clinical Oncology (ASCO)
- Learn more about TAPUR at https://www.tapur.org/
Genomics Evidence Neoplasia Information Exchange (GENIE)
- An international database that collects and links cancer genetic data with clinical outcomes from thousands of cancer patients, sponsored by the American Association for Cancer Research (AACR)
- Learn more about GENIE at https://www.aacr.org/professionals/research/aacr-project-genie/
Worldwide Innovative Networking in personalized cancer medicine (WIN)
- A network of 36 leading organizations in personalized cancer medicine covering 20 countries and 4 continents
- Learn more about WIN at http://winconsortium.org/
Applied Proteogenomics Organizational Learning and Outcomes (APOLLO)
- A collaboration between the National Cancer Institute (NCI), the U.S. Department of Defense (DoD), and the U.S. Department of Veterans Affairs (VA). Their goal is to learn more about which proteins are involved in certain diseases, such as cancer, and create new drugs that block these proteins.
- Learn more about APOLLO at https://proteomics.cancer.gov/programs/apollo-network
Myeloma – Developing Regimens Using Genomics (MyDRUG)
- A Multiple Myeloma Research Foundation (MMRF) study to learn how drugs approved (or close to approval) for other cancers work to treat myeloma (cancer in white blood cells)
- Learn more about MyDRUG at https://themmrf.org/we-are-curing-multiple-myeloma/mydrug/
-
How has the new approach helped?
While this approach would be costly for a single organization, the network approach has helped by sharing cost and patient data between many organizations. This helps researchers:
- Access more data on more patients than any researcher at a single organization could access alone
- Quickly find and match the right cancer patient to a cancer clinical trial for more treatment options
- Complete clinical trials more quickly
For patients, the new approach can discover new treatments to give them more treatment options. Overall, it helps to improve cancer care.